

· Long-acting, weekly intravenous fusion inhibitor, a chemically modified peptide that binds to serum albumin1,2
· Targets HIV gp41 envelope protein at entry point and inhibits the fusion of viruses with CD4+ T cells, thus blocking viruses from entering
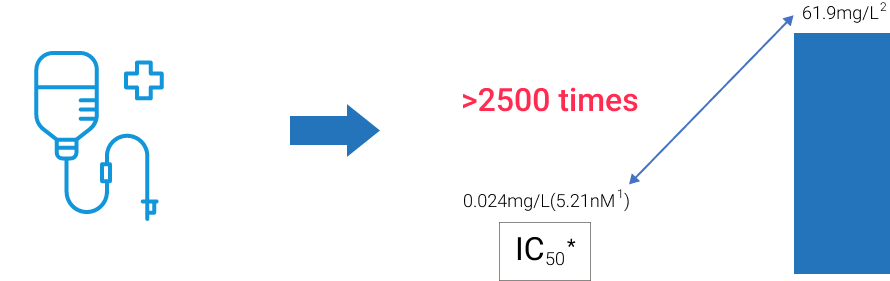
· High genetic barrier to resistance2,3
· Not a CYP450 enzyme inhibitor2
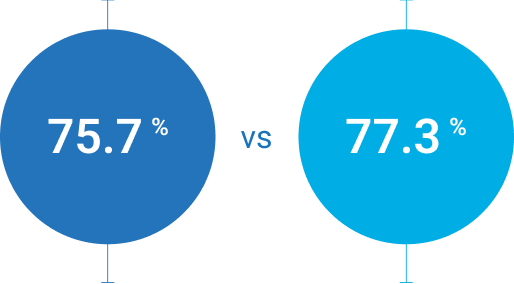
[95% CI: -10.1% -6.9%]
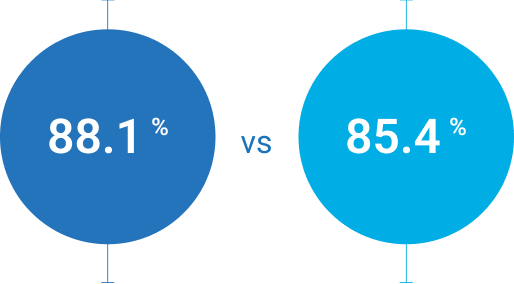
[95% CI: -10.1% -6.9%]
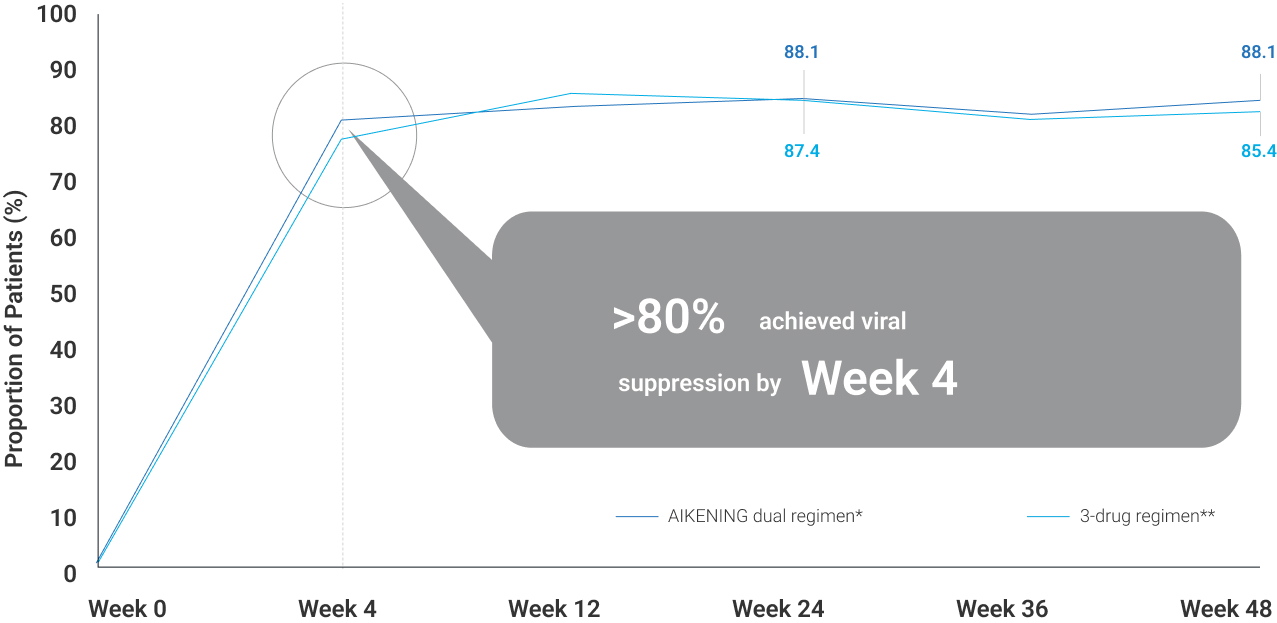
*AIKENING + LPV/r **2 NRTI + LPV/r
TALENT: Test Albuvirtide in Experienced Patients; PLHIV: people living with HIV;
HIV: human immunodeficiency virus; RNA: ribonucleic acid; CI: confidence interval; ARV:
antiretroviral; LPV/r: ritonavir-boosted lopinavir; NRTI: nucleoside reverse transcriptase
inhibitor
For adults and adolescents over 16 years old,AlKENING TM is administered by intravenous infusion or bolus injection at a dose of 320 mg once a day on Day 1,2,3.and 8, and thereafter once a week.
Administer AIKENINGTM solution by intravenous infusion via a peripheral vein at a rate of about 2 mL/min for 45±8 minutes.
Administer AlKENINGTM solution by intravenous bolus injection via a peripheral vein over no less than 30 seconds.
*IV bolus injection route of administration is approved in China.
1.Albuvirtide (Aikening) prescribing information
2.Chong H, et al. PLoS One.20212;7(3):e32599
3. Su B, et al. Chin Med J (Engl). 2020 Nov 25;133(24):2919-2927
4.Wu H, et al.Efficacy and safety of long acting HIV fusion inhibitor albuvirtide in treatment experienced HIV-1 infected patients: Week 48 analysis from the
randomized controlled phase 3 TALENT study. Presented at: 11th IAS Conference on HIV Science;18-21 July 2021; Poster Number PEB148
5.Su B, et al.J lnfect.2022 Sep;85(3):334-363 9. Davy-Mendez T, et al. J Infect Dis.2021June 15,223(12);2113-2123

Frontier Biotechnologies Inc.(Nanjing)
Copyright © 2016 Corporation All Rights ReservedFrontier Biotechnologies Inc.
