INDICATION
AIKENINGTM is a human immunodeficiency virus type 1 (HIV-1) fusion inhibitor indicated in combination with other antiretroviral agent(s) for the treatment of HIV-1 infection in treatment-experienced patients with HIV-1 replication despite ongoing antiretroviral therapy.¹
LONG-ACTING FUSION INHIBITOR
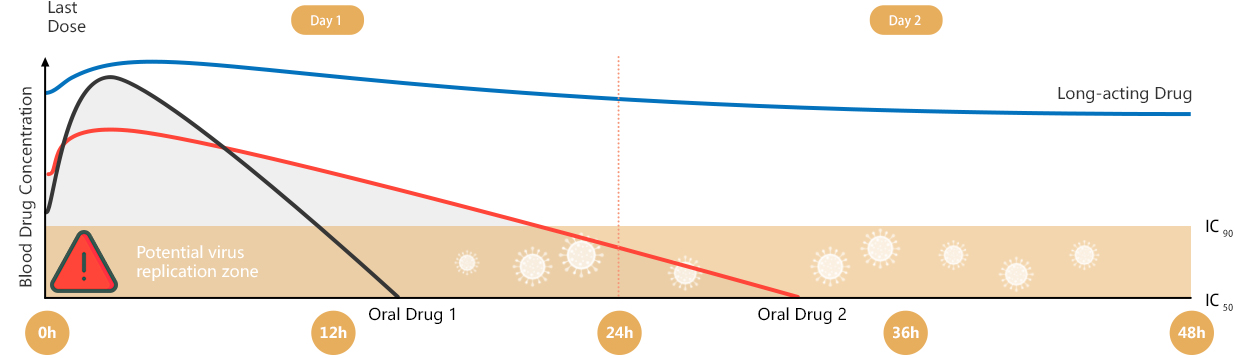
AIKENINGTM could ensure durable suppression of viral load and minimize the risk of HIV drug resistance, especially for people who have pill fatigue
The long-acting ability of AIKENINGTM is due to an incorporated 3-maleimidopropionic acid (MPA) group that specifically binds to and forms a stable conjugate with human serum albumin (HSA). The conjugate retains the biological activity of AIKENINGTM, and significantly prolongs its half-life.
As a long-acting antiretroviral therapy, AIKENINGTM may allow HIV-1-infected patients who have difficulty with daily oral therapy to maintain viral suppression within a relatively long dosing window. AIKENINGTM could ensure durable suppression of viral load and minimize the risk of HIV drug resistance, especially for people who have pill fatigue.
AIKENINGTM CLINICAL DATA
TRIAL Design
TALENT is a randomized, multicenter, open-label, phase 3 trial.2,3
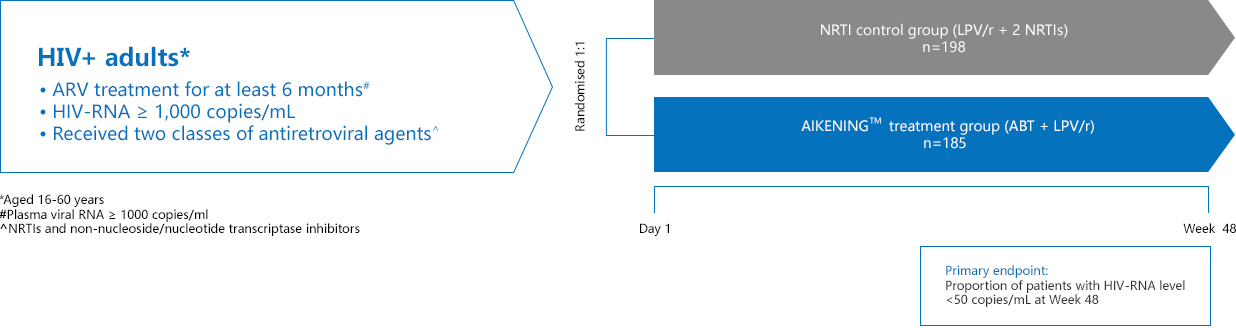
Primary endpoint: Proportion of patients with with plasma viral load (HIV-RNA) level<50 copies/mL at Week 48 by FDA snapshot algorithm
Secondary endpoints:
• Proportion of patients with plasma HIV-1 RNA level<400 copies/mL
• Changes from baseline
○ HIV-1 RNA log10 copies/mL
○ CD4+ T-cell counts
PATIENT BASELINE CHARACTERISTICS
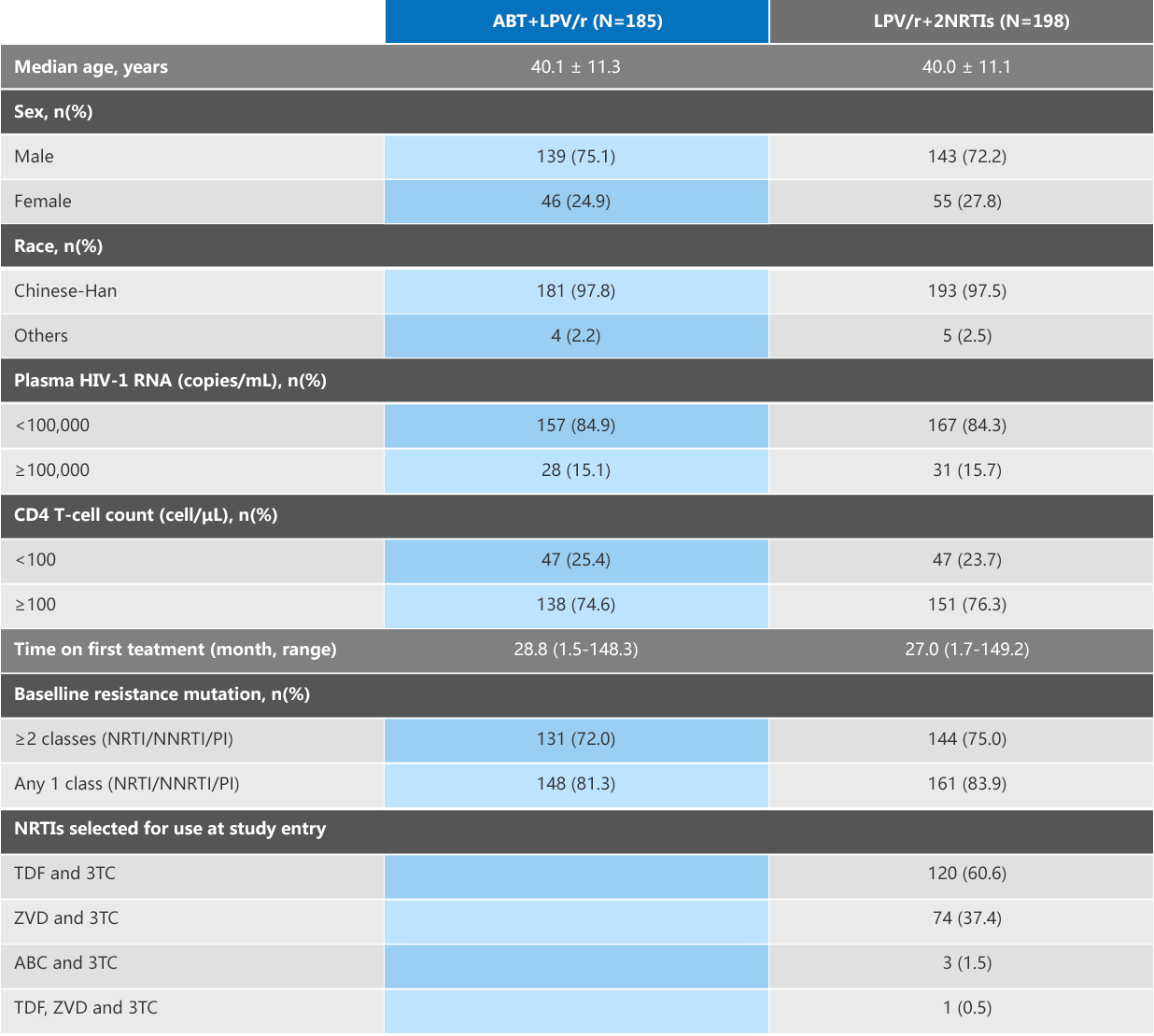
Of 374 subjects, the dominant viral subtypes were B (ABT group 49.5% vs. 2 NRTIs group 47.4%), CRF01_AE (32.4% vs. 36.5%), and CRF07_BC (8.2% vs. 3.1%).
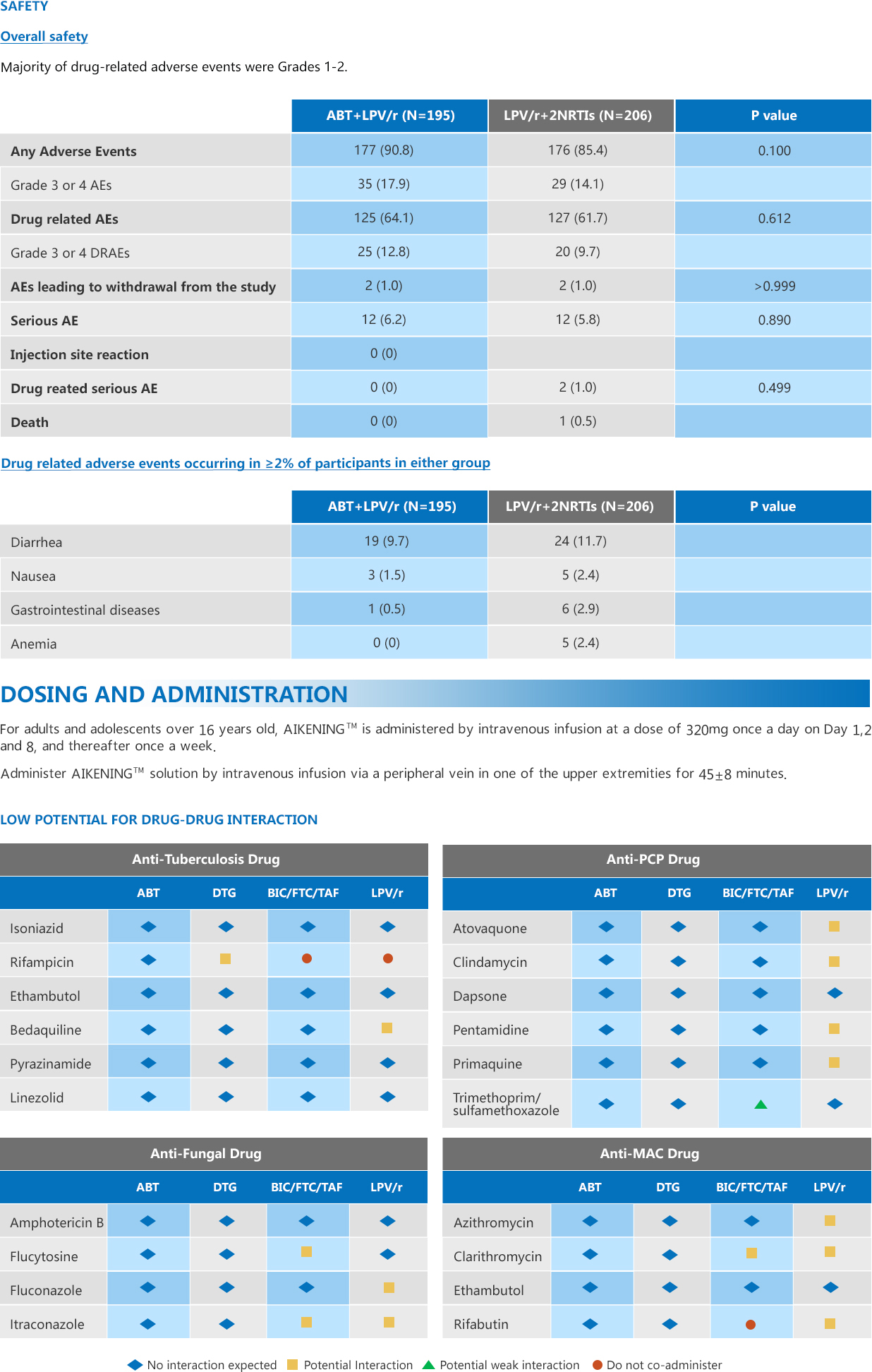
EFFICACY DATA
• AIKENINGTM was found to be non-inferior to a standard second line three drug regimen (upper bound of 95% CI for the treatment difference was<7%) at Week 48 for the primary endpoint (HIV-1 RNA ≤50 copies/mL).

• Similar incidence of confirmed virologic failure across both arms was observed at Week 48.**
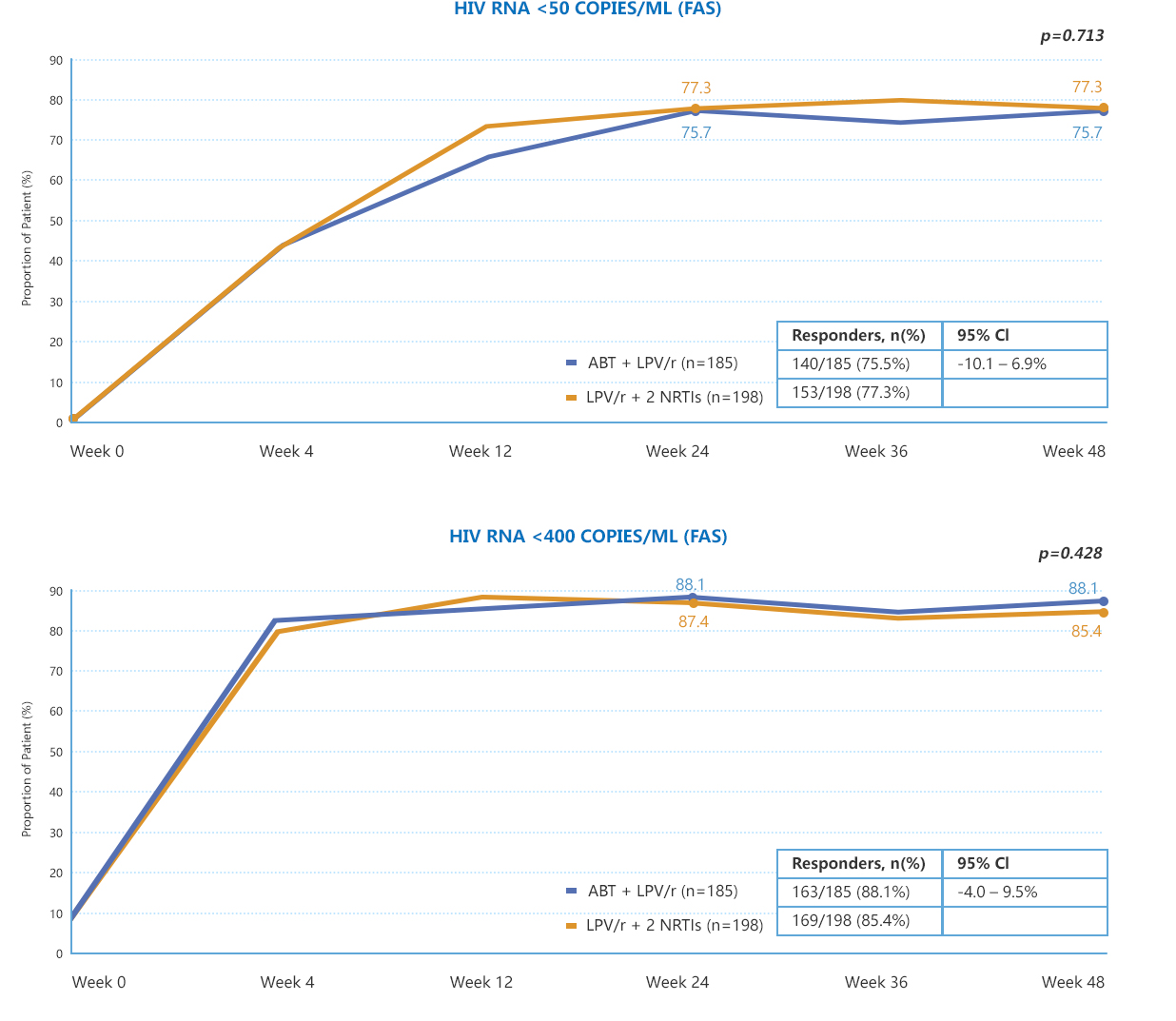
*In TALENT, the oral comparator consisted of 2 NRTIs + LPV/r.
**Virologic failure is defined as a confirmed viral load of more than 400 copies/mL.


• 1 of 11 patients had 1 new NRTI RAMs (K103S, G190A) at failure, but remained sensitive to NRTI and PI classes.
• 9 of 11 patients had no new RAMs.
Missing baseline drug resistance data in 1 participant, who had K103N, P225H and V82A at failure.
ABT = Albuvirtide; AZT = Zidovudine; BIC/FTC/TAF = Bictegravir/Emtricitabine/Tenofovir alafenamide; DTG = Dolutegravir; FDA = Food and Drug Administration; ITT = Intention-To-Treat; LPV/r = Lopinavir/ritonavir; MAC = Mycobacterium avium; PCP = Pneumocystis pneumonia; RAM=resistance-associated mutation; TDF = Tenofovir Disoproxil Fumarate; 3TC = Lamivudine; PP = Per Protocol.