In October 2021, in the “13th National AIDS and Hepatitis C Conference of the Chinese Medical Association”, organized by the AIDS Group of the Chinese Society of Infectious Diseases, Chinese Medical Association, the Chinese Guidelines for Diagnosis and Treatment of HIV/AIDS (2021 Edition) (hereinafter referred to as the 2021 Guidelines) was released. The first Guidelines for Diagnosis and Treatment of HIV/AIDS in China was formulated in 2005, led by the AIDS and Hepatitis C Group of the Chinese Society of Infectious Diseases, Chinese Medical Association. It was updated in 2011, 2015 and 2018 respectively. The 2021 Guidelines were based on the 4th Edition Guidelines in 2018 and revised with reference to the latest research progress in China and abroad.

Content of the 2021 Guidelines
The 2021 Guidelines fully updated the epidemiological and etiological characteristics of HIV and AIDS, clinical manifestations and stages, antiretroviral therapies for HIV , post-exposure prophylaxis treatment and management of the whole continuum of the HIV infection.
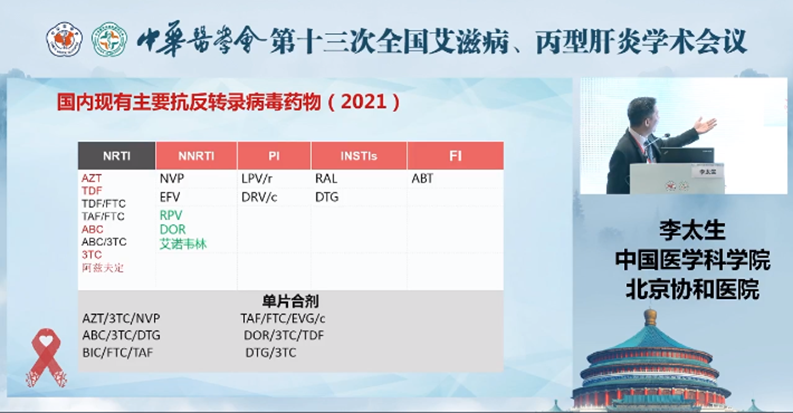
The key antiretroviral drugs in China
HIV post-exposure prophylaxis (HIV PEP) refers to people, who are not infected with HIV, receiving an anti-HIV blocking therapy as soon as possible (not exceeding 72 hours) after they are exposed to high HIV infection risks. It is one of the important strategies to reduce infection risks by active intervention.
The 2021 Guidelines further updated the antiretroviral drug list for HIV PEP and has included a new long-acting HIV fusion inhibitor, Aikening® (generic name: Albuvirtide/ ABT) as a recommended regimen for HIV PEP. Albuvirtide is a national Class 1 anti-HIV drug independently developed by Frontier Biotech (stock code: 688221.SH). It was marketed in 2018 and was listed in the Guidelines for Diagnosis and Treatment of HIV/AIDS in China (2018 Edition) as one of the key antiretroviral drugs available for use in China.
The world’s first long-acting HIV fusion inhibitor, Aikening® (generic name: Albuvirtide/ ABT).
Aikening® is a long-acting fusion inhibitor with HIV membrane protein gp41 as its target spot, consisting of 34 amino acids and one modified chemical group. It acts on the first link of HIV-1 infection, combines with the target spot, HIV-1 membrane protein gp41 and inhibits the fusion of viral membrane and CD4 cells of the human body, thus preventing HIV-1 from entering cells and eliciting an antiviral effect. Aikening® is bound with albumin in blood specifically through the side-chain modified group with the molecular ratio of 1:1, forming a stable conjugate field, thus resulting in a prolonged half-life which translates to a long-lasting effect. Aikening® targets gp41, which exists in all HIV-1 viruses it is in a highly conserved domain and is effective against the major epidemic HIV-1, including drug-resistant viruses. As a long-acting HIV fusion inhibitor, Aikening® binds to human serum albumin and inhibits HIV fusion, making it a favourable treatment option for HIV PEP. It constitutes an effective prophylaxis regimen against primary drug-resistant viruses and is of certain scarcity in the global HIV prophylaxis and treatment field. It provides an effective prophylaxis regimen even against drug-resistant viruses, thus addressing the specific needs in this area which currently has limited treatment options available.
On September 29, 2021, an article entitled Tolerability and Adherence of Antiretroviral Regimens Containing Long-Acting Fusion Inhibitor Albuvirtide for HIV Post-Exposure Prophylaxis: A Cohort Study in China was published in the international medical journal -- Infectious Diseases and Therapy. This clinical study is the world’s largest and China’s first prospective cohort study on the HIV PEP population to evaluate the safety, tolerability and adherence of the combination regimens of ABT with other oral drugs for HIV PEP. This study is supported by reliable sources of clinical studies and data collection, providing a clinical reference for evidence-based use in the treatment of HIV PEP.
The 2021 Guidelines emphasized HIV prevention, highlighting the importance of combining prevention, treatment, medication and prophylaxis in the treatment continuum.
HIV/AIDS is still a major public health concern. However, with good progress in prophylaxis and treatment of HIV/AIDS, coupled with the unceasing efforts of researchers pursuing scientific and technological advancements, a functional cure for HIV/AIDS could be in the near future.